


Our Story
About Globacure
Our Service
Services
Effective project management is crucial for the successful delivery of healthcare research and development projects. This encompasses initiating, planning, executing, monitoring, and closing projects to ensure they…
Product development in the biopharmaceutical industry requires a detailed, end-to-end strategy for transforming an idea into a tangible therapeutic solution. This process starts with initial…
Team Leaders
Our Special Team

Larry A. Walker, Ph.D.
President and CEO

Babu L. Tekwani, Ph.D.
Co-President & Chief Scientific Advisor

Fahim Ahmad, Ph.D.
Director Research and Development
Activites
Our Key Activies

Identifying Global Partners
Seeking Collaboration Opportunities with Research Institutions and Organizations to Drive Translational Advancements

Funding Opportunities
Initiating partnerships with with Funding Agencies and CROs to Accelerate Research and Development Efforts

Research Development Plans
Compiling Comprehensive Plans Outlining Critical Pathways for Effective Therapeutic Development

Botanicals Development
Facilitating the Regulatory Approval Process for Botanical Products in Various Countries

Regulatory Compliance
Ensuring Adherence to All Necessary Regulatory Frameworks for Research and Clinical Trials

Tailored Learning Programs
Developing Specialized Curricula for Workshops and Conferences to Educate Stakeholders
Current Pipeline
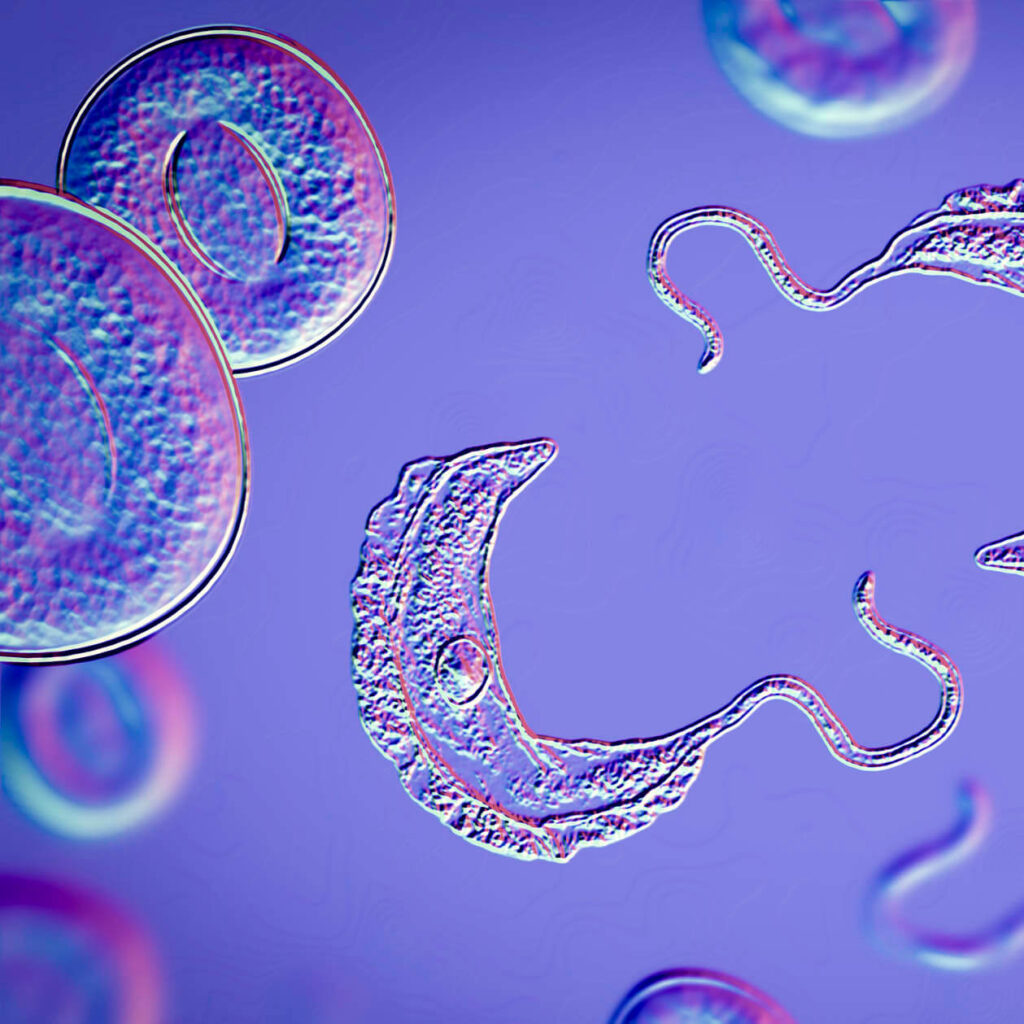
Neglected Tropical and Parasitic Infectious Diseases
Plasmodium vivax Malaria: Advancing clinical development for a radical cure that ensures safety in G6PD-deficient populations.
Malaria Prevention: Developing clinical strategies to prevent transmission of Plasmodium falciparum and Plasmodium vivax.
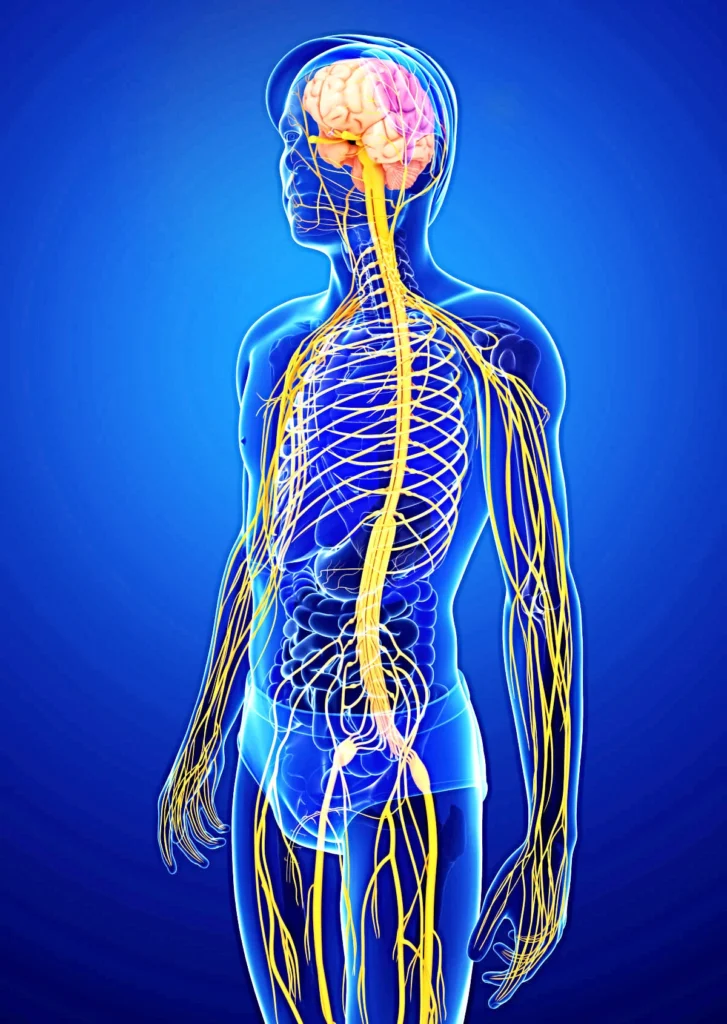
Neurological Diseases
Selective Natural Product Inhibitors: Exploring natural products that inhibit Human Monoamine Oxidase A and B in preclinical development.
Natural Products for Neuroprotection: Advancing formulations aimed at protecting against neurodegenerative processes in preclinical stages.
Individuals & Organizations
Partnerships

Researchers and Scientists

Healthcare Professionals and Practitioners

Non-Governmental Organizations (NGOs)
Government and Policy Makers

Pharmaceutical and Biotech Companies





